Children with LIS1 – Lissencephaly are born with a brain in which the typical furrows and convolutions are severely reduced or completely absent. This rare genetic disorder leads to severe developmental disorders, epileptic seizures and a limited life expectancy. For the families affected, the diagnosis often means great uncertainty, as the causes and progression of the disease are still poorly understood.
Organoid provides insight into the course of the disease
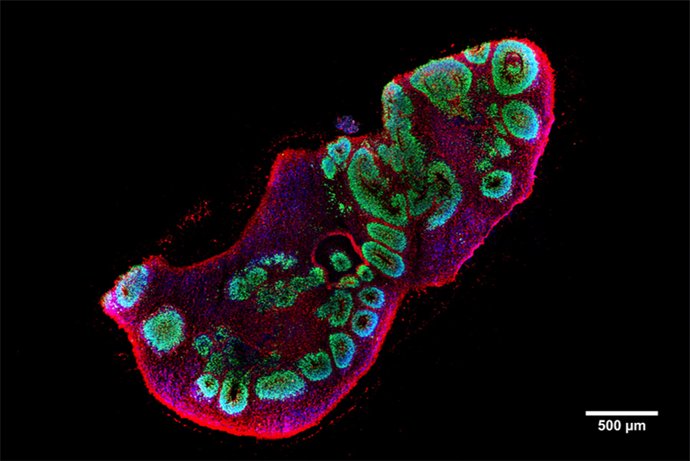
An international research team led by the Central Institute of Mental Health (CIMH) in Mannheim has now succeeded in developing a model for the disease in the form of so-called brain organoids – human brain tissue grown in the laboratory – in order to gain new insights into the development of LIS1 lissencephaly. The team led by CIMH researcher Dr. Julia Ladewig used stem cells from LIS1 patients to create three-dimensional brain organoids in the laboratory that mimic the early development of the cerebral cortex. This enabled the researchers to understand for the first time why mutations in the LIS1 gene cause different degrees of disease severity.
Cells retain their shape and stability thanks to an inner scaffold, the cytoskeleton. One of its essential components are so-called microtubules, tiny tubular structures inside the cell. The current study shows that the genetic changes in LIS1 destabilize the inner support structure of the cells, the microtubules. This disrupts the organization and division of nerve progenitor cells. These disorders can lead to the cell layers of the cerebral cortex being laid out irregularly – a central feature of the disease.
Detecting changes at the molecular level
The international team of researchers carried out single-cell RNA sequencing analyses. This method maps gene expression in thousands of individual cells. The researchers combined the knowledge of which genes are active in individual cells with data on the proteome, i.e. the totality of all proteins in the respective cells. This enabled them to record the disease-related changes at various molecular levels.
This approach showed that, in addition to the changes in the structure of the cytoskeleton, the cell proteins come under stress during the disease and can no longer maintain their normal folding – a process known as proteostasis dysregulation. This mechanism could be a common factor in many neurodevelopmental disorders. “The proteomic analyses helped us to better understand the functional consequences of the gene alterations and to identify molecular vulnerabilities,” explains Dr. Matteo Gasparotto, researcher at the Hector Institute for Translational Brain Research (HITBR) at the CIMH and one of the first authors of the study.
Available medication could help
The team then searched for known active substances that influence these disturbed signaling pathways. This showed that the already clinically approved drug everolimus, among others, was able to partially restore the cellular balance in the organoids. “This is an exciting indication that already available drugs could also help with rare developmental disorders,” says Dr. Lea Zillich, researcher at HITBR and first author of the study.
“Our organoid models allow us to observe the disease at a very early stage at the cellular level and directly compare the effects of different mutations,” explains Dr. Julia Ladewig, head of the Developmental Brain Pathologies research group at the CIMH and last author of the study. “This helps us to understand why some children are more severely affected than others,” she adds.
Contribution to the reduction of animal testing
The researchers emphasize that the use of organoid technology can also help to reduce animal testing, as it enables processes of human brain development to be studied directly in the cell culture dish and the efficacy of drugs to be tested.
The work was carried out in close collaboration with international partners and was published in the journal Nature Communications. It underlines how patient-derived organoids can help to understand individual disease severity and develop personalized therapeutic approaches.
Publication
Zillich L. et al. (2025) Capturing disease severity in LIS1-lissencephaly reveals proteostasis dysregulation in patient-derived forebrain organoids. Nature Communications (2025). DOI: 10.1038/s41467-025-64980-0
https://www.nature.com/articles/s41467-025-64980-0